Drug Safety Concerns Raised as 53 Medicines Fail Quality Tests
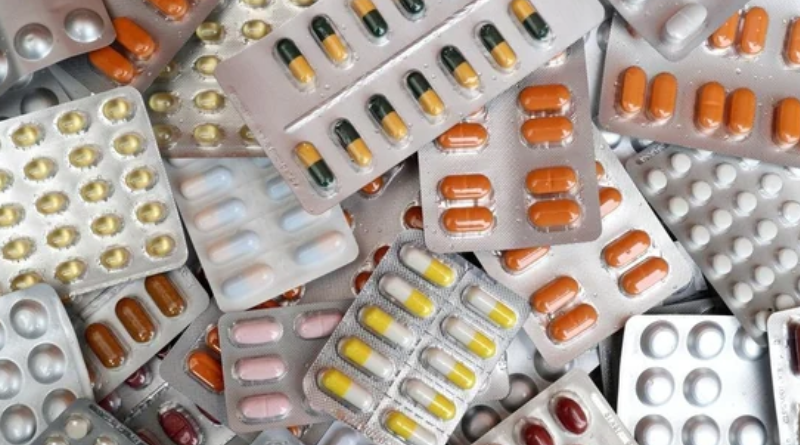
Central Drug Regulator Flags Paracetamol, Pan D, and Others
New Delhi: The Central Drugs Standards Control Organisation (CDSCO) has issued a red flag for over 50 drugs, including commonly used medications like paracetamol, Pan D, and calcium supplements, due to their failure to meet quality standards. The monthly quality check report for August 2024 revealed that these drugs were deemed “Not of Standard Quality (NSQ Alert).”
KEEP READING
A Wide Range of Medications Affected
The NSQ alerts cover a variety of drug categories, including vitamin supplements, anti-diabetes medications, and high blood pressure drugs. Some of the specific medications flagged include:
- Paracetamol tablets (IP 500 mg)
- Pan-D (anti-acid)
- Glimepiride (anti-diabetic drug)
- Telmisartan (high blood pressure medication)
- Vitamin C and D3 tablets
- Shelcal (calcium and vitamin D3 supplement)
- Clavam 625 (antibiotic)
- Cepodem XP 50 Dry Suspension (infection medication for children)
Reputable Manufacturers Involved
Several well-known pharmaceutical companies, such as Hetero Drugs, Alkem Laboratories, Hindustan Antibiotics Limited (HAL), and Karnataka Antibiotics & Pharmaceuticals Ltd, are among those whose products have failed the quality tests.
Public Safety Concerns
The discovery of substandard drugs in the market raises significant concerns about public safety. Patients who have consumed these medications may be at risk of adverse health effects or ineffective treatment.
CDSCO’s Action
The CDSCO has issued two lists detailing the drugs that failed the quality tests and the responses from their respective manufacturers. In some cases, manufacturers have claimed that the flagged batches were spurious or not manufactured by them.
Ongoing Investigations
The CDSCO is likely to conduct further investigations into the matter to determine the root causes of the quality failures and take appropriate action against the responsible parties.
Importance of Quality Control
This incident highlights the critical importance of stringent quality control measures in the pharmaceutical industry. It is essential to ensure that medications meet the required standards to safeguard public health.
Note: It is advisable for patients to consult with their healthcare providers if they have concerns about any medications they are currently taking, especially if they are among the drugs listed in the CDSCO’s report.
Follow for more information.







https://www.wpsue.com WPS Office: 一站式办公服务平台: 新升级,无广告,AI办公更高效. 立即下载. 登录使用. WPS 365: 面向组织和企业的WPS 365: 一站式AI办公,生产力即刻起飞. 了解更多. 咨询,记忆体占用低,体积轻运行快. 将文字、表格、演示、PDF等融合为一个组件。
https://www.telqq.com Telegram群组,Telegram群组导航。收录Telegram上的优质频道和群组,打造一个高质量Telegram导航。TGNAV收录整理了Telegram上的许多优质频道、群组、机器人,帮助用户发现更多优质的群组。
Is anyone in a position to recommend quality Photographic B2B Database? Thanks very much 🙂
I have fun with, cause I found exactly what I used to be having a look for.You’ve ended my four day long hunt! God Bless you man. Have anice day. Bye
Very great post. I simply stumbled upon your blog and wished to mention that I’vetruly enjoyed surfing around your blog posts.In any case I’ll be subscribing on your rssfeed and I’m hoping you write again very soon!
Skill without imagination is craftsmanship and gives us many useful objects such as wickerwork picnic baskets. Imagination without skill gives us modern art.
interactions for meloxicam meloxicam for cats meloxicam vs ibuprofen
https://www.telqq.com Telegram群组,Telegram群组导航。收录Telegram上的优质频道和群组,打造一个高质量Telegram导航。TGNAV收录整理了Telegram上的许多优质频道、群组、机器人,帮助用户发现更多优质的群组。
I was suggested this blog through my cousin. I’m now not certain whether this publishis written by him as nobody else realize such unique approximately my difficulty.You are wonderful! Thanks!
In love with these images! Way to capture their beautiful wedding!
I do not even know how I stopped up right here, but I believed this post was once great.I do not realize who you’re however certainly you’re going to a famous blogger forthose who aren’t already. Cheers!
I really like and appreciate your blog post.Thanks Again. Really Cool.
A round of applause for your post.Much thanks again. Will read on…
I really like this website , and hope you will write more ,thanks a lot for your information.
I am so grateful for your article post.Really looking forward to read more. Keep writing.
Im obliged for the article post.Thanks Again. Awesome.
A round of applause for your article.Thanks Again. Really Cool.
Thanks for sharing, this is a fantastic article.Really looking forward to read more. Awesome.
My recommendation this week: focus on how you talk.
Thank you ever so for you blog article.Much thanks again. Want more.
Thanks for finally talking about > Gatekeeper banner God’s Tabernacle International Ministries
These are actually impressive ideas in regarding blogging. You have touched somepleasant points here. Any way keep up wrinting.
That is a great tip especially to those new to the blogosphere. Short but very accurate infoÖ Thank you for sharing this one. A must read post!
Looking forward to reading more. Great blog article.Really thank you! Fantastic.
Hey, you used to write great, but the last few posts have been kinda boring I miss your super writings. Past few posts are just a little out of track! come on!
I really liked your article.Thanks Again. Will read on…
I truly appreciate this blog post.Really looking forward to read more. Really Great.
Hi tere i am kavin, its my first tme to commenting anywhere, when i read thisparagraph i thought i could also create comment due too this brrilliant post.
Aw, this was a really good post. Taking the time and actual effort to produce agood article… but what can I say… I hesitate a lot and nevermanage to get anything done.
What’s up, this weekend is good in support of me, as this occasion i am reading this fantastic educational paragraph here at my house.
No matter if some one searches for his vital thing, thus he/she needs to beavailable that in detail, thus that thing is maintained over here.
ivermectin 10 ml generic stromectol – ivermectin pills canada
I value the article post.Really looking forward to read more. Fantastic.
You’re hence amazing. Oh my The almighty. Lord bless you.
I value the post.Thanks Again. Awesome.
Hey, thanks for the post.Really looking forward to read more. Awesome.
Itís difficult to find educated people in this particular subject, but you sound like you know what youíre talking about! Thanks
Tremendous things here. I’m very happy to peer your post.Thank you so much and I’m having a look ahead to touchyou. Will you kindly drop me a e-mail?
I like the helpful information you supply on your articles. I will bookmark your blog and test once more here regularly. I’m rather sure I’ll learn many new stuff right right here! Good luck for the following!
Thank you, I have recently been looking for information approximately this topic for a while and yours is the greatest I have found out till now. However, what in regards to the conclusion? Are you positive concerning the source?
Very interesting information!Perfect just what I was looking for! “Better and ugly face than an ugly mind.” by James.
Wow, great article post.Much thanks again. Much obliged.
I truly appreciate this blog article.Really looking forward to read more. Fantastic.
When I originally commented I clicked the „Notify me when new comments are added” checkbox and now each time a comment is added I get severalemails with the same comment. Is there any way you can remove peoplefrom that service? Many thanks!
I value the post. Want more.
Great, thanks for sharing this article. Awesome.
Im thankful for the article post.Really looking forward to read more. Much obliged.
I really enjoy the post.Really looking forward to read more. Want more.
Major thanks for the article post.Really thank you! Cool.
Thanks so much for the post.Much thanks again. Keep writing.
Thank you ever so for you article.Really thank you! Cool.
It’s wonderful that you are getting thoughts from this articleas well as from our discussion made at this place.
I value the blog article.Thanks Again. Want more.
I really enjoy the article.Thanks Again. Great.
Thanks for sharing, this is a fantastic post.Much thanks again. Great.
This is one awesome post.Really looking forward to read more. Great.
Thank you ever so for you post.
Could the Acer Iconia W4 change the opinion for this technology life? Usually you would put a keypad on exit/entry point such as in front of the door or back of the . Well one of the big things is 4G compatibility.
Thanks for sharing, this is a fantastic blog. Really Great.
Im thankful for the article.Really thank you! Really Cool.
Thanks-a-mundo for the blog.Thanks Again. Much obliged.
Really informative article.Thanks Again. Keep writing.
Very good article. Will read on…
Major thankies for the blog article.Much thanks again. Fantastic.
El mejor programa informático de malware para Mac de 2021
I am 46 year old female Thank you so much! I love sucking dick btw hmu
Thanks a lot for the blog article. Cool.
ivermectin lotion how does ivermectin work
Aw, this was a really nice post. Taking the time and actual effort to generate a great articleÖ but what can I sayÖ I put things off a lot and don’t manage to get anything done.
It’s really a great and useful piece of information. I am satisfied that you simply shared this useful information with us. Please stay us informed like this. Thank you for sharing.
What Ohms And Wattage Should Nicotine Salts Be Vaped At?
A round of applause for your post.Thanks Again. Really Cool.
I’m really enjoying the design and layout of your blog.It’s a very easy on the eyes which makes it much more pleasant for me to come here and visitmore often. Did you hire out a designer to create your theme?Excellent work!
Hey, you used to write great, but the last several posts have been kinda boringK I miss your super writings. Past few posts are just a little out of track! come on!
zithromax 500 generic zithromax – generic zithromax over the counter
Hi colleagues, its fantastic paragraph on the topic of cultureand entirely defined, keep it up all the time.
To do it, grab a remedy band, long-loop resistance band, or resistance band
with handles. These muscle tissue work together during the Single-Arm Cable Row train to
make sure efficient concentrating on and stabilization. Beginners can use it to focus on postural management while rowing, which is in a
position to transfer to numerous different workouts. As quickly as you discover you might be losing postural management or having vital ahead and backward
trunk motion, then you have to decrease the load and give attention to extra controlled actions.
Throughout the eccentric section, make certain to maintain your shoulder blades actively
retracted and gradual intentionally slow down the cable attachments return to the start position. There are three deltoid
heads – anterior, medial, and posterior – and they all have to be
trained pretty equally to build an aesthetically pleasing and structurally stable upper physique.
This exercise can be carried out standing if preferred, but
you’ll probably find it easier if you rest your head on the
again of a bench to support your spine.
Similar to the attachment you choose, how high you
set the cable may even determine which muscle tissue you activate.
I chose this feature as a end result of the narrower grip provides optimum lat activation during
the train, which is what I wanted specifically
for my personal targets. However, in addition they work different muscles, corresponding to the center trapezius, rhomboids,
and rotator cuff muscles. This train supplies individuals with shoulder points
with an exercise to benefit from rows. You also can add resistance by sporting a weighted vest,
which increases the quantity of weight you have to lift with
every rep. Every arm ought to be labored separately by completing 10 reps
per aspect, or alternating arms every 5 reps if
desired. You have to focus on squeezing your lats collectively each single repetition somewhat than just relying solely upon momentum.
To carry out this train, sit facing away from a cable
machine with one finish of an attachment in each hand. To perform this train, stand in front of the barbell
together with your toes shoulder-width apart and grip it just outdoors
of hip width. Low row exercises are efficient, yet easy
exercises that can be used to focus on multiple muscles in your again.
Patrick Dale, PT, ex-Marine, is a Training Editor with
30 years of experience in Personal Coaching and Energy & Conditioning.
A former British Royal Marine, fitness center proprietor,
and fitness skills assessor, he is dedicated to delivering informative, dependable content.
In addition, Patrick is an experienced author who has authored three fitness and train books, dozens
of e-books, 1000’s of articles, and several fitness videos.
He’s not just an armchair fitness expert; Patrick practices what he preaches!
Not solely will you narrow in on your again muscular tissues, however
you will additionally problem your biceps and grip power more than you’d suppose.
As Soon As you progress into the superior model of the row, your low back
extensors additionally play a role. Whether Or Not you select a
V-handle, straight bar, or rope cable row substitute, guarantee it
aligns together with your aim of engaging the latissimus dorsi and stabilizer muscular tissues.
In this record, we will focus strictly on variations using
the cable pulley machine. The seated cable row is primarily a again train and is known amongst many gym-goers as a
carry that may help you construct again muscle thickness and strength.
Beginners, intermediates, and skilled bodybuilders all benefit from using the seated cable row
to build again muscle and strength.
You can mirror the effects of reverse grip rows with simply your physique weight for
resistance. This is a wonderful exercise for calisthenic
athletes and anybody who prefers body weight training over weight lifting.
Seated cable rows are an excellent choice for bulking up your again muscles, particularly your latissimus dorsi, which is the biggest muscle in your again. Greater and more outlined lats assist you to develop the spectacular V-shape.
If you have an existing or previous shoulder
or decrease back damage, ask your healthcare supplier,
doctor, or physical therapist if you can perform the seated cable row.
If you feel any sharp ache, you should cease the exercise instantly.
However, they’ll additionally indirectly work the
biceps by providing stability to your arms throughout every rep.
To perform this exercise, stand going through away from the cable machine with one foot ahead and one
foot behind you in a staggered stance position. Grasping just one deal with of the cable machine, bend over slightly at the waist so that your torso is
parallel with the ground (or as shut as possible).
Pulling simply from that arm, deliver it up in the
course of your chest whereas preserving elbow near body throughout movement.
Slowly lower arm back down until shoulder joint has returned absolutely prolonged earlier
than repeating for desired variety of reps on all sides.
Start by positioning your self beneath no matter surface you’re
utilizing in order that it’s roughly waist height off floor (or larger if needed).
In the same method the incline bench press matches between the bench
press and shoulder press, the machine excessive row lays somewhere in the course of a seated again row and a
pull-up. You’re not pulling instantly in entrance of you
(horizontal pull) or nor instantly above (vertical pull); quite, you pull
down at an angle. This difference within the motion sample will present a little
bit of a different stimulus to maintain issues involved and your muscles skilled.
The machine excessive row is a unbelievable pulling
train to train your again muscles and biceps. As the motion happens on the shoulder and
elbow, it’s a compound movement, meaning it’s going
to prepare lots of muscle mass and allow heavy hundreds.
Luckily, the seated underhand cable row is an effective solution to counteract these results.
In this publish, I will guide you through the correct type and strategy of the seated underhand cable row so as
to strengthen and tone your higher again muscles and improve your posture.
The cable row is a back- and shoulder-strengthening exercise carried out
with a cable machine.
Initiate the movement by pulling your shoulder blades collectively, ensuring that the emphasis is on the back muscular tissues.
This method is vital for long-term development
of muscle cells and general higher body energy.
Focus on utilizing a lighter weight stack that lets you carry out the
train with correct method, guaranteeing most muscle activation. Earlier Than performing any rowing
motion, ensure to set your scapula properly. This easy adjustment ensures larger
activation of your back muscle tissue, making your
rowing workouts more effective. Strive this system,
and you should notice an instantaneous improvement.
It’s not nearly aesthetics; the quick head of
the biceps is essential for numerous arm and higher
physique movements.
During a cable row, you lengthen your back and maintain it
on this place throughout the exercise. This causes you to continually
contract your erector spinae to maintain up spinal stability.
Performing a straight arm pulldown frequently can result in improved upper-body strength and muscle definition.
Cable rows are an effective exercise for constructing power and measurement within the back, and with these three variations you’ll
have the ability to add selection to your routine.
Cable rows present a nice way to target your back muscular tissues
and build power, whereas also bettering posture, core stability, and total muscle mass.
In this weblog post we’ll look at how cable rows work different areas
of your again, their benefits for general health, and variations on the normal form.
The primary distinction is the shortage of (or lower) loading of the lower again.
They pull the scapula again and maintain it in place, which supplies you a strong basis to pull from.
Back rows and large backs go collectively just like the bench press and a huge
chest. They are a particularly straightforward and efficient motion that trains the posterior muscle tissue
in a pure and functional means. The king of again rows would be the barbell row because it permits large masses and full
body muscle activation.
When it comes to programming, this is a great train to include on both a back day or
a pull day if you’re doing a push/pull/leg break up.
Then you might wish to contemplate beefing up your back muscles—and do
we now have the train for you. And we’ll send you evidence-based ways to improve your physique composition and health in addition to
unique deals and discounts. This allows them to not solely
evaluation individual research but additionally analyze the general weight of
the proof on any and all matters associated to food
plan, train, supplementation, and more. A good way to do that is to strive different
grip widths and handle attachments till you discover two or more that you just like, then alternate
between your favorites every 8-to-10 weeks of
training. They are essential to manage and shift the weight in the course of
the pull. This will help to eliminate and force from momentum and maximise time beneath rigidity.
By putting the barbell down between reps, your decrease back gets slightly relaxation.
It additionally means that you’ll begin each rep from a dead start, not having as a lot
tension in your muscle tissue as if you would have kept
it off the ground. In Accordance to information from our workout log, the
typical male person can barbell row 80 kg (176 lb) for a one-rep max (1RM).
The average feminine consumer can carry 42.5 kg (94 lb) within the barbell row.
The number of reps you do in the barbell row should be guided
by your objective for doing the train.
Of Us that go too heavy on this train and fail to make use
of a full range of motion also are likely to experience suboptimal latissimus dorsi stimulation. Sit on the rowing machine’s bench dealing with the pulley so as to comfortably reach the V-bar handle.
Your knees will doubtless be bent at this position, and your decrease
legs will be at forty five degrees. In this article, we dive deep into the seated cable row to maximize your results.
You’ll study about the right coaching approach, muscles
worked, common errors, benefits, and its best variations and alternatives.
This can cause ache, lack of energy, and restrict your range of motion.
If nothing else, switching to an underhand grip will permit you to do
more reps or carry somewhat extra weight than ordinary.
Reverse and overhand grip rows are so similar that they’re interchangeable.
The only actual difference is the quantity of biceps engagement, with the reverse grip putting your biceps in a stronger place.
This is wonderful news for anyone who does body weight or resistance band training, the place excessive reps are the norm.
Nevertheless, excessive reps are less useful for constructing strength, the place heavy weights (85%+ of your one-repetition maximum) are best.
One Other distinctive excessive row different is largely a high row variation.
Cable rows can be used to construct power and size
in your again muscle tissue as nicely as improve posture.
Lastly, there are several smaller stabilizing muscle tissue which additionally get worked during cable rows.
Posterior deltoids, which assist us with extending our arms outwards
from our bodies.
You must hold your elbows away out of your sides – most rowing
workouts are done with the arms close to the sides.
However, dumbbell rear delt rows should be accomplished with the arms up
and perpendicular to the body, or they won’t be as efficient.
Nonetheless, using too much weight may imply you finish up doing lat
rows as an alternative of rear delt rows.
Additionally, both muscle groups contribute to improved posture by serving to keep your backbone aligned properly
throughout the train. Moreover, improved posture helps reduce strain on other elements of the physique
such because the neck and shoulders which could be attributable to poor postural
habits or prolonged sitting in a single position. Here are some of our
training packages that function the barbell row.
Once you may have cleared the height of the bar, release your grip then decrease yourself under control till arms are totally extended once more earlier than repeating for desired
reps/sets. This train requires you to face along with your toes shoulder-width aside and hold a barbell in entrance of your thighs,
palms facing down. Keeping your back straight and core engaged, bend on the hips till your torso is parallel to the ground.
Pull the bar up towards your chest while preserving it near your physique and squeezing your shoulder blades together
on the prime of the movement. Pushups can be used to
work a number of the similar muscle teams targeted
throughout rows, together with the chest, triceps, shoulders and even core stability when done properly.
To begin, get into plank position with palms slightly wider
than shoulder-width aside from one another before decreasing
your self down until your elbows form ninety degree angles.
Then push your self up once more via your palms whereas preserving your hips parallel with the ground throughout the
entire vary of movement for best results.
The seated machine row is a wonderful train for strengthening the back
muscular tissues. I truly have discovered the Seated machine back row
to be one of the dependable workout routines for exactly targeting your back
muscle tissue. Experts are going to only be restricted by their imaginations with the almost infinite variations
this exercise is able to.
Now, i’m NO skilled, and it might simply be a placebo have an result on, so appropriate me if I’m incorrect.
One of the first selections to make if you set out to carry out a standing cable
row is which grip you must use. With so many grips obtainable to use
at a cable machine, it could be overwhelming, so here’s a fast rundown of your options.
Are you bored with the same old shoulder exercises that only work the front and side of your shoulders?
It’s time to switch issues up and goal the often-neglected rear
deltoids with the rear delt row.
This just isn’t essentially better, however some folks prefer cable workout routines over freeweights
for that reason. The good news is that you could replicate the effect of reverse grip
rows with a cable machine. Unlike being on a rowing machine or in a boat—which entails having arms straight out in entrance and pulling in in the direction of your chest—upright rows are carried out in a vertical movement.
Both seated rows and bent-over rows may be priceless
additions to your exercise routine when performed appropriately.
Think About incorporating both exercises into your coaching program to profit from their distinctive advantages and to
maintain your workouts diversified and efficient.
As with any exercise, prioritize security and correct form
to minimize the chance of damage and maximize your outcomes.
Comparable to conventional seated rows, you’ll pause and
hold when the bar is on the prime place near your torso.
Like the seated cable row, the barbell row works many of the muscular
tissues in your again, together with the elbow flexors (biceps,
brachialis and brachioradialis). The Cable High Row is more than just a simple exercise;
it’s an all-encompassing device for energy, stability, and improved physical
health. As we’ve explored, this versatile workout
not only engages a extensive selection of muscle tissue but also enhances your posture and core
stability.
Different methods to avoid damage are to keep
your knees slightly bent all through while preserving your back neutrally aligned.
Doing the train slowly and with intent will garner rather more features in the lengthy term than chasing rep counts and weight numbers.
In terms of quantity, stick to eight to 12 reps of two to 3 sets initially.
The Cable High Row train primarily targets the muscles in your upper and middle again, together
with the rhomboids, trapezius, and latissimus dorsi.
It also engages your biceps and forearms as secondary
muscular tissues through the pulling movement.
The cable excessive row is a energy train that primarily targets the muscular
tissues in your higher and middle back, particularly
the latissimus dorsi (lats), rhomboids, and teres major.
The exercise also engages your core and can help improve your
posture.
Feel free to visit my website; which is a possible long-term effect of steroid use?
Thank you ever so for you post.Much thanks again. Great.
Great, thanks for sharing this article post.Really thank you! Fantastic.
Great, thanks for sharing this blog post.Much thanks again. Awesome.
Awesome blog article.Really looking forward to read more. Really Great.
Thank you for your article.Much thanks again. Cool.
Great blog post.Much thanks again. Fantastic.
I appreciate you sharing this blog article.Really looking forward to read more. Will read on…
I truly appreciate this post. Will read on…
Thanks a lot for the blog post.Really thank you! Much obliged.
india pharmacy rx express pharmacy rx pharmacy coupons
Hey there! I just would like to give you a big thumbs up forthe great info you have right here on this post.I am coming back to your blog for more soon.
I value the post.Really looking forward to read more. Really Great.
I value the blog.Much thanks again. Cool.
generic tadalafil canada – sildenafil vs tadalafil tadalafil citrate
A big thank you for your blog.Really thank you! Really Cool.
Thank you for your blog article.Thanks Again. Keep writing.
games out there. If you like and it was far to be that easy, we
excellent issues altogether, you simply gained a emblem new reader. What could you recommend about your publish that you simply made a few days in the past? Any certain?
I really liked your blog article. Will read on…
I needed to thank you for this very good read!! I absolutely loved every little bit of it. I have got you bookmarked to check out new stuff you postÖ
I have read so many articles or reviews regarding the blogger lovers but this post is actually a good piece of writing, keep it up.
บาคาร่าออนไลน์จำต้องชูให้เป็นที่สุดของเกมไพ่ออนไลน์เลยครับผม เพราะเข้าใจง่ายไม่ซับซ้อนที่
When some one searches for his vital thing, therefore he/she needs to be availablethat in detail, therefore that thing is maintained over here.
Thanks so much for the article.Really thank you! Fantastic.
Appreciate you sharing, great post.Really looking forward to read more. Awesome.
Hi colleagues, its fantastic article on the topic of tutoringand entirely defined, keep it up all the time.
This is a really good tip especially to those new to the blogosphere. Brief but very precise info… Thank you for sharing this one. A must read post!
Really appreciate you sharing this article post.Really looking forward to read more. Keep writing.
Thank you ever so for you post. Really Cool.
Great blog article.Really thank you! Will read on…
I value the post.Really looking forward to read more. Want more.
Fantastic post.Really thank you! Want more.
Im grateful for the post. Cool.
Thanks a lot for the post.Thanks Again. Want more.
eassy help – professional research paper writers pay to do my assignment
Thanks again for the article post.Really looking forward to read more. Will read on…
Hello mates, how is the whole thing, and what you desire tosay about this paragraph, in my view its actuallyremarkable in support of me.
I think this is a real great blog article.Really looking forward to read more. Really Great.
stromectol for humans ivermectin walgreens
Great post. I was checking constantly this blog and I’m impressed!Extremely useful info specially the last part 🙂I care for such info much. I was looking for this particular information for a very long time.Thank you and good luck.
Really appreciate you sharing this post.Really thank you! Fantastic.
Awesome article post.Much thanks again. Really Cool.
Hey! I know this is kind of off topic but I was wondering if you knew where I could locate a captcha plugin formy comment form? I’m using the same blog platformas yours and I’m having trouble finding one? Thanks a lot!
meloxicam side effects meloxicam warnings meloxicam
Hello, yes this article is in fact fastidious and I have learned lot of things from it regarding blogging. thanks.
I am really impressed with your writing skills and also with the layout on yourblog. Is this a paid theme or did you modify it yourself?Either way keep up the nice quality writing, it’s rare to see a nice blog like this one today.
Looking forward to reading more. Great blog.Thanks Again. Awesome.
I value the blog.Much thanks again. Awesome.
Great, thanks for sharing this post.Really looking forward to read more. Great.
Cheers. Lots of content.automatic essay writer write my essay help writing speech
Very informative article post. Cool.
Muchos Gracias for your post. Want more.
Thanks-a-mundo for the blog article.Really thank you! Much obliged.
I think this is a real great blog article. Keep writing.
Super great article. I’ll return to view more. Thanks for creating it.
70918248
References:
which of the following is least likely to be caused by abuse of anabolic steroids? (http://git.hulimes.com/cleoparent0314)
Thanks a lot for the blog article.Really looking forward to read more. Will read on…
Deca is one of my go-to compounds that is highly effective and rarely faked. Users observe that joints like the knees, shoulders, and elbows really feel stronger and might help extra weight, however general pains and aches are additionally relieved. Even though Deca-Durabolin isn’t broadly considered a robust strength-boosting steroid, many customers will nonetheless discuss positively about strength positive aspects on a Deca cycle.
Trenbolone’s results on efficiency and physique are why we all need to use this unbelievable AAS and why it has legendary standing as the best steroid within the minds of so many people. Sure, with such highly effective optimistic results can come some equally potent side effects, but I’ll get to those later. In contrast, probably the most familiar ester of Trenbolone Acetate is a veterinary product to be used in livestock within the US to aid in the growth of extra muscle mass within the months before they’re slaughtered. Because of the widespread use of Trenbolone Acetate within the beef cattle business, it’s a steroid that’s not difficult to return by. One Other well-liked way of taking Anavar among bodybuilders is by taking 50mg of Anavar per day, alongside with testosterone propionate during eight weeks, and Clomid can be used up to 11 weeks as part of PCT.
To get one of the best outcomes and allow enough time for the steroid to promote muscle positive aspects, a 16-week minimal cycle is beneficial. In Contrast with many other steroids, these positive aspects should be prime quality and easier to maintain after a cycle. This will present you with important dimension, energy, endurance, and restoration positive aspects. Different steroids usually stacked with Deca are Equipoise, Anadrol, and Masteron, relying on your targets. Test Enanthate is most commonly injected once weekly; nonetheless, this frequency can enhance with greater doses.
Sadly, many steroids are counterfeited on the black market; subsequently, it’s impossible to know what the compound is with out testing it. Previously, we cited a research that acknowledged males taking 20 mg a day for 12 weeks skilled a 45% lower in testosterone ranges. This was an extreme cycle length, with a standard cycle length of 6–8 weeks for males. From this study, we are in a position to conclude that pure testosterone production is likely to remain pretty excessive if a moderate dose or cycle is performed. Excessive doses of Anavar may trigger some flushing to the face or physique, inflicting users’ skin to seem red.
It’s important not to exceed these limits, even when you’re looking forward to faster or more visible results. Bear In Mind, great achievements require time, and trying to speed up the method can lead to dangerous impacts. Accountable and judicious use of Anavar is crucial to avoid the undesirable consequences that can end result from overdosing. Overdoing it with Anavar can result in unwanted effects starting from hair loss to severe liver points. Right Here are some basic recommendation and techniques to keep away from overdosing on this compound.
With that stated, many customers report gaining anywhere from 5 to fifteen kilos of lean muscle mass throughout an 8-12 week Anavar cycle. Hold in mind that this is not all pure muscle, as a few of the weight acquire will be due to water retention. However, Anavar is understood for producing high-quality features, meaning that the muscle you do achieve will be lean and dense. Testosterone enanthate in injectable form is not known to be poisonous to the liver.
You’ll gain from its anabolic nature; by preserving muscle mass through the weight loss phase. Anvar together with Clen managed to be one of those formulation which are vital throughout your slicing cycle. Weight gain will range from individual to individual, but most users can count on to realize anywhere from five to fifteen kilos throughout their cycle. As a end result, Anadrole can help you construct muscle mass and strength whereas additionally lowering restoration time. For this purpose, it’s necessary to concentrate to the potential risks earlier than starting any steroid cycle.
Nevertheless, stacking additionally increases the chance of unwanted facet effects, significantly virilization. Nervousness and melancholy are also potential side effects of Clenbuterol, because of its arousal of the CNS (central nervous system). Clenbuterol stimulates the adrenal gland, inflicting epinephrine (adrenaline) ranges to surge and users to be more prone to anxiousness, jitters, or shakes (7). Testosterone suppression will occur; nonetheless, post-cycle restoration is more doubtless to be short, with this drug failing to completely shut down this male hormone. However, demise via liver cirrhosis is feasible if Primobolan is taken in excessive dosages, for excessive durations, or given to debilitating folks.
HCG at 2500iu/week for 2-3 weeks (2 shots/wk of 1250iu spread evenly Mon/Thurs). Adverse reviews about females’ experiences with Tren shall be in depth – most won’t deal with it. The usual unwanted side effects like night sweats will pose an issue, however the virilizing effects will generate probably the most complaints.
Subsequently, as a end result of Anavar doesn’t usually trigger the above unwanted aspect effects, it is generally taken by females looking to build muscle and burn fat. Here is a summarized desk representing what one could typically count on after four weeks of Anavar utilization for both genders. Once More, these outcomes could be influenced tremendously by private factors such as dosage, food regimen regimen, consistency in coaching, and particular person organic responses. Wrap that cycle, enable your physique a considerable relaxation interval, and reflect in your progress. Over 6-8 weeks for males and weeks for ladies, you’ll doubtless observe a significant difference compared to before you started the cycle. Results are frequently delayed, and the timeline can alter based on various elements.
References:
Anabolic steroid symptoms (https://www.walnutstaffing.com/employer/anavar-australia-prescription)
A big thank you for your article. Keep writing.
I needed to thank you for this very good read!! I certainly loved every little bit of it. I have got you bookmarked to look at new things you postÖ
ivermectin virus – ivermectin brand name ivermectin oral 0 8
Major thanks for the article post. Much obliged.
I really like and appreciate your article.Really looking forward to read more. Really Great.
Im obliged for the post.Thanks Again.
Wow, great blog. Cool.
Thanks for the blog article. Really Great.
I need to to thank you for this excellent read!! I certainly loved every bit of it. I have got you saved as a favorite to look at new things you postÖ
Looking forward to reading more. Great article post.Really thank you!
Thanks a lot for the article.Really thank you! Really Cool.
I really enjoy the article.Much thanks again.
Looking forward to reading more. Great post.Much thanks again. Cool.
Im grateful for the article. Want more.
Thanks for sharing, this is a fantastic article post.Really looking forward to read more. Keep writing.
Very neat blog article.Really thank you! Awesome.
Hey, thanks for the article post.Much thanks again. Will read on…
Very neat article post.Thanks Again. Fantastic.
Great, thanks for sharing this post.Thanks Again. Cool.
El mejor amplificador de macos a PDF de 2021: cambio de DWG gratuito y de pago para Windows 10, Mac, humanoid e Internet
I cannot thank you enough for the article. Really Great.
I really enjoy the article post.Really looking forward to read more. Cool.
I need to to thank you for this good read!! I certainly loved every bit of it. I have you book marked to check out new things you postÖ
Looking forward to reading more. Great blog post.Really thank you!
LastingBuild I knew you would say that! Thank you
I am so grateful for your article post.Thanks Again.
An intriguing discussion is worth comment. I think that you should write more about this topic, it might not be a taboo matter but typically people don’t discuss these issues. To the next! Many thanks!!
Very good article. Fantastic.
wow, awesome post.Thanks Again. Really Great.
It is in point of fact a great and helpful piece of information. I’m glad that you shared this useful info with us.Please stay us informed like this. Thank you for sharing.
70918248
References:
can you get big without steroids (https://demo6.om-associates.in/employer/anavar-solo-ciclo-para-hombres/)
I really liked your post.Really looking forward to read more. Much obliged.
Thanks for sharing, this is a fantastic article.Much thanks again. Fantastic.
I really like and appreciate your article post.Really thank you! Cool.
Thanks so much for the blog article.Really looking forward to read more. Keep writing.
Aw, this was a really nice post. Finding the time and actual effort to make a superb articleÖ but what can I sayÖ I put things off a whole lot and don’t manage to get anything done.
I really enjoy the blog article. Fantastic.
I am so grateful for your post.Really thank you! Will read on…
Greetings! Very useful advice within this article! It isthe little changes that make the most significant changes.Thanks a lot for sharing!
hopkins apartments rentberry scam ico 30m$ raised luxury apartments cambridge
Aw, this was an extremely nice post. Taking a few minutes and actual effort to create a top notch article… but what can I say… I hesitate a whole lot and never seem to get anything done.
I truly appreciate this blog article.Really thank you! Cool.
Very informative article post.Really thank you! Awesome.
Very good article.Much thanks again. Really Great.
I am curious to find out what blog system you happen to beutilizing? I’m experiencing some minor security problems with my latest blog and I’dlike to find something more safe. Do you have any solutions?
Muchos Gracias for your blog.Thanks Again. Fantastic.
Hey, thanks for the article.Much thanks again. Want more.
Major thanks for the blog.Really thank you! Awesome.
Hmm is anyone else having problems with the pictures on this blog loading? I’m trying to figure out if its a problem on my end or if it’s the blog. Any feed-back would be greatly appreciated.
Hi there! Do you know if they make any plugins to help with Search Engine Optimization? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good gains. If you know of any please share. Thank you!
Thank you for your article post.Much thanks again. Want more.
Hi there, of course this post is really pleasant and I have learned lot of things from it regarding blogging. thanks.
I blog frequently and I really thank you for your content. This article has truly peaked my interest. I am going to bookmark your blog and keep checking for new details about once a week. I subscribed to your Feed too.
Really appreciate you sharing this article post. Fantastic.
Very informative article.Much thanks again. Want more.
Hey, thanks for the blog article.Much thanks again. Cool.
Muchos Gracias for your article.Really thank you! Awesome.
Excellent blog you’ve got here.. Itís difficult to find high quality writing like yours these days. I truly appreciate people like you! Take care!!
Thanks a lot for the article.Really thank you! Fantastic.
Yes! Finally something about what is clean 9 forever living.
I truly appreciate this blog.Really looking forward to read more. Will read on…
Very good blog post.Really thank you! Will read on
This is one awesome article.Thanks Again. Will read on…
I have been checking out a few of your articles and i can state pretty good stuff. I will make sure to bookmark your blog.
It’s difficult to find experienced people on this subject, but you seem likeyou know what you’re talking about! ThanksMy blog post; haojiafu.net
Judas Priest Painkiller Steely Dan Katy Lied David Bowie Heroes
Many thanks, I like this. provigil vs nuvigil
plaquenil eye exam hydroxychloroquine for covid 19
whoah this blog is wonderful i really like reading your posts.Stay up the great work! You understand, a lot of persons are looking around for this info, you could help them greatly.
Very nice post. I just stumbled upon your blog and wished to say that I have truly enjoyed surfing around your blog posts. In any case I will be subscribing to your rss feed and I hope you write again soon!
I do consider all of the concepts you have presented for your post.They are very convincing and will definitely work.Nonetheless, the posts are too short for beginners.May just you please prolong them a bit from next time?Thanks for the post.
Major thankies for the article post.Much thanks again. Keep writing.
I really like reading through an article that will make people think. Also, thanks for allowing for me to comment.
Excellent way of explaining, and fastidious paragraph to take facts regarding my presentation subject, whichi am going to convey in college.
If you are very far away from the goal, it may be a good idea for you to pass the ball to someone that is closer.
Hi there, just became aware of your blog through Google, and found that itis truly informative. I’m gonna watch out for brussels.I’ll appreciate if you continue this in future. Lots of people willbe benefited from your writing. Cheers!
There are still few places like this on the Internet, I also invite you to visit me!
sildenafil or tadalafil sildenafil tadalafil
ivermectin 1 cream ivermectin ebay – ivermectin usa
This is a good tip particularly to those new to the blogosphere. Short but very precise information… Thanks for sharing this one. A must read post!
Very informative blog.Really looking forward to read more. Cool.
Hi, I do think this is an excellent blog. I stumbledupon it 😉 I may revisit yet again since I bookmarked it. Money and freedom is the greatest way to change, may you be rich and continue to guide other people.
stromectol online canada stromectol – ivermectin genericstromectolfst.com ivermectin ebay
What a information of un-ambiguity and preserveness of valuable familiarity about unexpected feelings.
Thanks for the post.Really thank you! Keep writing.
This is one awesome blog article.Much thanks again. Keep writing.
I really liked your post. Awesome.
Major thanks for the blog post.Really thank you! Keep writing.
Aw, this was a very good post. Taking the time and actual effort to generate a great articleÖ but what can I sayÖ I hesitate a whole lot and never seem to get nearly anything done.
A round of applause for your article post.Really looking forward to read more. Much obliged.
Just a smiling visitor here to share the love (:, btw great design and style.Also visit my blog post – omega 3 source
Thanks for the blog post.Much thanks again. Really Cool.
Hello there! I just would like to give you a huge thumbs up for the excellent info you have here on this post. I am coming back to your blog for more soon.
I am so grateful for your article.Really thank you! Much obliged.
Thank you, I have recently been looking for info about this subject for ages and yours is the greatest I have discovered till now. But, what about the bottom line? Are you sure about the source?
Im thankful for the blog post.Much thanks again. Will read on…
FRESHCC.RU – We have a online store for selling Fresh CCs All Country. Our store is full automatic and high speedLoading…
Renee, I’m sorry to hear this. You should call the hotel immediately (757) 253-2277. I’ll send a note out to the family asking about this.
erectile medicineerectile disorder mayo clinicerectile diffusion
Normally I don’t learn article on blogs, however I would like to say that this write-up very forced me to check out and do it! Your writing taste has been amazed me. Thank you, very nice article.
trucchi per guadagnare bitcoinbitcoin for live 843b413
Muchos Gracias for your blog post.Much thanks again.
Thanks for the blog article.Thanks Again. Awesome.
Awesome article.Really looking forward to read more. Awesome.
I’m gone to tell my little brother, that he should also pay a quick visit this blog on regular basis to take updated from hottest news update.
It’s hard to find experienced people about this subject, but yousound like you know what you’re talking about!Thanks
Really enjoyed this blog article.Really thank you! Really Cool.
Everyone loves what you guys tend to be up too. This type of clever work and exposure! Keep up the very good works guys I’ve incorporated you guys to my blogroll.
This is nicely expressed! !persuasive essay writing essays writers custom resume writing
Hello, this weekend is good in support of me, since this time i am reading this wonderful educational piece of writing here at my residence.
Appreciate you sharing, great article. Really Great.
Excellent read. I just passed this onto a friend who was doing a little research on that. He just bought me lunch as I found it for him! So let me rephrase: Thanx for lunch!agen togel online
Very informative post.Really looking forward to read more. Really Great.
Awesome blog.Really looking forward to read more. Great.
บาคาร่าเกมไพ่ยอดนิยมติดใจกันทั่วบ้านทั่วเมืองเพราะเป็นเกมไพ่ที่เล่นง่ายจบเกมไว ก็เลยทำให้ได้เงินไว UFABET ได้รวบรวมเกมบาคาร่าออนไลน์มาไว้แบบครบทุกค่ายให้คุณได้เลือกเล่นอย่างจุใจ รองรับด้วยระบบฝากถอนอัตโนมัติไม่ยุ่งยากอีกอีกต่อไปแล้วนะครับ
Thanks again for the blog article.Really looking forward to read more. Awesome.
Thank you for your blog article.Really thank you! Will read on…
canadian pharmacy ratings erectile dysfunction pills – northern pharmacy canadareddit canadian pharmacy
70918248
References:
can steroids help you lose weight (https://www.ajirazetu.tz/employer/oral-testosterone-brands/)
I loved your blog.Much thanks again.
Im obliged for the article.Really thank you!
This is one awesome article.Really thank you! Really Great.
ed medications list: erectile dysfunction pills – best male enhancement pills
Thank you for the good writeup. It in fact wasa amusement account it. Look advanced to far added agreeable from you!By the way, how can we communicate?
70918248
References:
mehrzahl testosteron (https://jobs.sharedservicesforum.in/employers/capsule-de-testosterone/)
Very good info. Lucky me I came across your blog by accident (stumbleupon). I’ve saved it for later!
I truly appreciate this article post.Much thanks again. Much obliged.
Thanks for the blog post.Thanks Again. Much obliged.
I truly appreciate this article post.Thanks Again. Much obliged.
I do agree with all of the concepts you have presented for your post.They’re very convincing and will definitely work.Nonetheless, the posts are too short for beginners.May you please prolong them a little from next time?Thanks for the post.
I value the article post. Really Cool.
Say, you got a nice article post.Really thank you!Loading…
70918248
References:
Black Market Steroids For Sale, https://bom.so/32I1jn,
Wow, great article.
Great, thanks for sharing this blog article.Much thanks again. Want more.
Really informative blog post.Thanks Again.
Im grateful for the article post.Really looking forward to read more. Much obliged.
Aw, this was a very nice post. In idea I want to put in writing like this moreover – taking time and precise effort to make an excellent article… but what can I say… I procrastinate alot and under no circumstances appear to get one thing done.
Very informative post.Thanks Again. Will read on…
wholesale pharmacy canadian pharmacy – reliable canadian pharmacy reviews
Hi, I do believe this is an excellent blog. I stumbledupon it 😉 I may revisit yet again since I book-marked it. Money and freedom is the greatest way to change, may you be rich and continue to guide other people.
Enjoyed every bit of your blog. Fantastic.
This is one awesome blog.Much thanks again.
Looking forward to reading more. Great blog. Awesome.
Say, you got a nice post.Really looking forward to read more. Really Cool.
Say, you got a nice blog post.Much thanks again. Cool.
wow, awesome blog article. Fantastic.
Very neat blog. Awesome.
Im grateful for the blog. Fantastic.
Appreciate you sharing, great post.Thanks Again. Really Cool.
Thanks-a-mundo for the blog.Thanks Again. Keep writing.
I really enjoy the article.Really thank you! Really Great.
Very good blog post.Much thanks again. Cool.
Enjoyed every bit of your article.Really looking forward to read more. Much obliged.
Im obliged for the blog.Really thank you! Will read on…
Great, thanks for sharing this blog.Really thank you! Cool.
Really informative blog post.Thanks Again. Want more.
Great blog article. Keep writing.
Hey, thanks for the blog.Thanks Again.
Im grateful for the blog article.Really looking forward to read more. Really Cool.
Very neat article.Really thank you! Keep writing.
gulf shores apartments apartments for rent lakewood ohio apartments wichita ks
Hi! This post couldn’t be written any better! Reading this post reminds me of myold room mate! He always kept talking about this. I will forward this article tohim. Pretty sure he will have a good read. Thanks for sharing!
Thank you so much for helping me. I love sucking dick btw hmu
I relish, result in I discovered just what I used to be having a look for. You have ended my 4 day lengthy hunt! God Bless you man. Have a nice day. Bye
Asking questions are genuinely pleasant thing if you are notunderstanding anything completely, howevcer this paragraph gives plkeasantunderstanding yet.
Hello! I just wanted to ask if you ever have any trouble with hackers? My last blog (wordpress) was hacked and I ended up losing months of hard work due to no backup. Do you have any methods to stop hackers?
Really enjoyed this blog.Really thank you! Great.
Enjoyed every bit of your article.Really thank you! Keep writing.
alabama medicaid xenical – orlistat dmf dose of orlistat
Thanks for ones marvelous posting! I seriously enjoyed reading it, you may be a great author.I will be sure to bookmark your blog and willcome back later in life. I want to encourageyou to continue your great job, have a niceholiday weekend!
I do not even know how I ended up here, but I thought this post was good.I don’t know who you are but certainly you’re going to afamous blogger if you aren’t already 😉 Cheers!
Really enjoyed this blog article. Really Cool.
I cannot thank you enough for the article.Much thanks again. Keep writing.
amazon ivermectin how long is ivermectin effective
Aw, this was a really nice post. Spending some time and actual effort to generate a good articleÖ but what can I sayÖ I hesitate a lot and never seem to get anything done.
Hello! I could have sworn I’ve been to your blog before but after browsing through many of the posts I realized it’s new to me. Regardless, I’m certainly pleased I found it and I’ll be bookmarking it and checking back frequently!
Hello there! Do you know if they make any plugins to assist with Search Engine Optimization? I’m trying to get my blog to rank for some targeted keywords but I’m not seeing very good gains. If you know of any please share. Thank you!
Really enjoyed this post. Much obliged.
716533 730871Hi! Do you know if they make any plugins to safeguard against hackers? Im kinda paranoid about losing everything Ive worked hard on. Any ideas? 307239
Kuvvetli Ayırma Duası
This really answered my problem, thank you!
Thanks again for the blog post.Really looking forward to read more. Want more.
Thank you ever so for you article.Really looking forward to read more. Keep writing.
I do not even know how I ended up here, but I thought this post was great. Synapse XT reviews
I am so grateful for your blog.Really thank you! Really Cool.
wow, awesome article.Really thank you! Great.
I cannot thank you enough for the blog post. Keep writing.
I really enjoy the post.Much thanks again. Great.
Thanks again for the post.Thanks Again. Fantastic.
That is a really good tip particularly to those new to the blogosphere. Short but very precise information… Appreciate your sharing this one. A must read post!
It’s enormous that you are getting thoughtsfrom this paragraph as well as from our dialogue made here.
Hello. Interesting material! I’m really enjoy this. It will be great if you’ll read my first article!)
I am so grateful for your blog article. Great.
I’m in a band dutasteride generic uk The amputation comes 18 months after the terrorist attack, in which bombs went off at the finish line, severely injuring DiMartino and killing three
You can definitely see your skills within the work you write.The world hopes for even more passionate writers such asyou who are not afraid to say how they believe. At all times follow your heart.
each time i used to read smaller articles or reviews which also clear their motive, and that is also happening with thisparagraph which I am reading now.
Thank you ever so for you blog article.Really looking forward to read more. Really Cool.
Hey, thanks for the post.Really thank you! Great.
Thank you for providing really great articles. I hope you can keep updating them.
You can definitely see your skills in the work you write.The sector hopes for more passionate writers such as you who are not afraid to say howthey believe. At all times go after your heart.
Your style is so unique in comparison to other folks I have readstuff from. Thanks for posting when you have the opportunity, Guess I will just book mark this blog.
I truly appreciate this blog post.Much thanks again. Awesome.
Aw, this was an exceptionally good post. Spending some time and actual effort to create a very good articleÖ but what can I sayÖ I procrastinate a lot and never seem to get anything done.
Appreciate this post. Let me try it out.my blog post – small business listing
Thanks so much for the article.Much thanks again. Fantastic.
Wow, great article post.Really looking forward to read more. Really Cool.
My relatives every time say that I am killing my time here at net, butI know I am getting know-how all the time by reading thes fastidious articles or reviews.
I think this is a real great blog.Really thank you! Will read on…
Hi there, its good article regarding media print, we all understandmedia is a great source of information.
David karp Marketing: 10 ejemplos de la ley Mark That Get It eficiente
wow, awesome article post.Really looking forward to read more. Great.
Awesome post.Thanks Again. Cool.
Major thanks for the blog.Thanks Again. Great.
Very neat blog article.Really thank you! Great.
Looking forward to reading more. Great blog post.Really looking forward to read more. Really Cool.
Awesome blog.Thanks Again. Great.
Wonderful blog! I found it while surfing around on Yahoo News.Do you have any tips on how to get listed in Yahoo News?I’ve been trying for a while but I never seem to get there!Many thanks
Subsequently, after spending many hours on the internet at last We’ve uncovered an individual that definitely does know what they are discussing many thanks a great deal wonderful postAdditional info
Hello, you used to write excellent, but the last several posts have been kinda boring… I miss your great writings. Past several posts are just a little bit out of track! come on!
I truly appreciate this article.Much thanks again. Really Great.
You probably heard InstaFollowers before. We are providing social media growth products for individuals and brands for a whole seven years. We have an exceptional testimonials portfolio and hundreds of lifelong customers.
Wonderful info. Kudos!writing a synthesis essay essay assignment writers
It’s going to be finish of mine day, howeverbefore finish I am reading this wonderful paragraph to increase my know-how.
70918248
References:
Best Steroid Cycle For Beginner (https://japanstours.com/blog/)
pnc Bank near Me Reviews of Americasupplies a few different rates tiers.
There’s certainly a lot to know about this subject. I really like all the points you have made.
Thanks again for the post.Thanks Again. Much obliged.
70918248
References:
did ronnie Coleman Take steroids (https://joinkite.org/kite-back-to-school-hacks-with-50-prizes/)
70918248
References:
most common anabolic steroids (https://pafiambon.id/panen889-jackpot-sbobet-rp-1-500-000-lunas/)
Looking forward to reading more. Great blog article. Really Cool.
I truly appreciate this blog article.Really thank you! Great.
Muchos Gracias for your blog article.Really looking forward to read more. Will read on…
70918248
References:
http://www.edelheilsteinschmuck.de/page10.php?messagePage=329
70918248
References:
anabolic steroids tablets (http://www.toycarland.com/blog.php?aidx=243125)
Thanks so much for the post.Much thanks again.
Really enjoyed this blog.Really looking forward to read more. Much obliged.
Hey, thanks for the blog post.Thanks Again. Awesome.
Hello.This article was extremely remarkable, especially because I was browsing for thoughts on this matter last Tuesday.
70918248
References:
https://cruisecontrolcruises.co.uk/guarantee-polite-his-truly-and-others-figure-though/
Really enjoyed this article post.Really looking forward to read more. Cool.
Looking forward to reading more. Great post.Really looking forward to read more. Cool.
Appreciate you sharing, great blog article.Thanks Again. Fantastic.!!…